A progression toward hybrid and electric cars seems like a natural step in the evolution of automobile technology. Analysts aren't predicting a drop in oil and gasoline prices anytime soon, effectively shifting car manufacturers into high gear to find the next best way to power vehicles. If the recent concept cars revealed by major automakers are accurate predictors, Lithium-ion batteries (Li-ion) may be that magic bullet.
Take, for instance, the Chevrolet Volt. This is General Motors' plug-in hybrid concept car designed to run for 40-mile (64.3-kilometer) stretches entirely off of Li-ion batteries. After that, a small gas engine will take over for another 600 miles (965 kilometers). That means many people could complete their daily commute without burning a drop of gas. In addition, the company plans to start cranking them out en masse by 2010.
Advertisement

And Chevrolet isn't alone in the Li-ion trend. Jeep, Cadillac, Dodge, Land Rover, Chrysler and Saturn all previewed 2008 concept cars that feature Li-ion battery packs for greener driving [source: Mahoney].
Why this fawning over Li-ion batteries in the first place? The Toyota Prius and two new hybrids the company unveiled in June 2008 use a nickel-metal-hydride battery. According to the most recent EPA standards, the Prius gets a combined 46 miles per gallon -- not to mention that it has sold like hotcakes. [source: fueleconomy.gov].
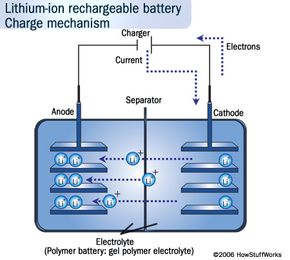
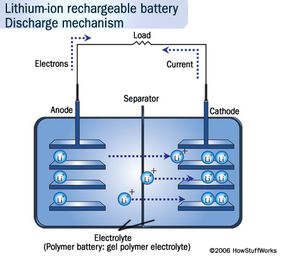
But in energy terms, Li-ion batteries simply pack a more powerful punch. Li-ion batteries store more energy in smaller spaces than the more traditional lead-acid and nickel-metal-hydride ones. Lithium has the most energy density and electrochemical potential of all metals, which is what gives it that stamina [source: Buchmann]. The nickel-metal-hydride batteries in hybrids on the road are also heavy, limiting their potential, whereas Li-ion batteries can amp the speed without weighing the car down. Because of this property, you can find smaller versions of them in many consumer electronics products, such as ]laptops, cell phones and iPods.
But there are a few bumps in the road for Li-ion batteries to become tomorrow's gasoline, namely safety, costs and longevity. We'll check out the safety issues on the next page.
Advertisement