The fuel cell will compete with many other energy conversion devices, including the gas turbine in your city's power plant, the gasoline engine in your car and the battery in your laptop. Combustion engines like the turbine and the gasoline engine burn fuels and use the pressure created by the expansion of the gases to do mechanical work. Batteries convert chemical energy back into electrical energy when needed. Fuel cells should do both tasks more efficiently.
A fuel cell provides a DC (direct current) voltage that can be used to power motors, lights or any number of electrical appliances.
There are several different types of fuel cells, each using a different chemistry. Fuel cells are usually classified by their operating temperature and the type of electrolyte they use. Some types of fuel cells work well for use in stationary power generation plants. Others may be useful for small portable applications or for powering cars. The main types of fuel cells include:
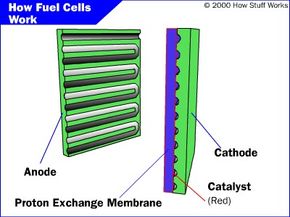
Polymer Electrolyte Membrane Fuel Cell (PEMFC)
The PEMFC, also known as the proton exchange membrane fuel cells, has a high power density and a relatively low operating temperature (ranging from 60 to 80 degrees Celsius, or 140 to 176 degrees Fahrenheit). The low operating temperature means that it doesn't take very long for the fuel cell to warm up and begin generating electricity.
Solid Oxide Fuel Cell (SOFC)
Factories or towns could receive their electricity from solid oxide fuel cells, which work best with large-scale stationary power generators. Fuel cells of this kind operate at very high temperatures (between 700 and 1,000 degrees Celsius). This high temperature makes reliability a problem because parts of the fuel cell can break down after cycling on and off repeatedly.
However, solid oxide fuel cells are very stable when in continuous use. In fact, the SOFC has demonstrated the longest operating life of any fuel cell under certain operating conditions. The high temperature also has an advantage: The steam that the fuel cells produce can be channeled into turbines to generate more electricity. This process is called co-generation of heat and power (CHP) and it improves the overall efficiency of the system.
Alkaline Fuel Cell (AFC)
This is one of the oldest designs for fuel cells; this type was the first the United States space program used to produce electricity and drinkable water on-board rockets and shuttles. NASA favored this method because the alkaline cell has high efficiency and a low operating temperature. However, because it’s highly susceptible to contamination, it requires pure hydrogen and oxygen. And it is unlikely to be commercialized because of the high cost.
Molten Carbonate Fuel Cell (MCFC)
Like the SOFC, these fuel cells are also best suited for large, stationary power generators. They operate at over 600 degrees Celsius, so they can generate steam that can be used to generate more power. The cell uses a molten salt solution as a catalyst that separates hydrogen particles from a traditional fuel like natural gas.
This process means that the fuel cell doesn’t require external refining equipment, but the high operating temperature also makes it susceptible to corrosion. They have a lower operating temperature than solid oxide fuel cells, which means they don't need such exotic materials. This makes the design a little less expensive.
Phosphoric-acid Fuel Cell (PAFC)
The phosphoric-acid fuel cell has been reliably used for the longest amount of time when it comes to hydrogen energy technology. This type of cell sees use in stationary power generation as well as industrial vehicles and buses. It operates at a higher temperature than proton exchange membrane fuel cells, so it has a longer warm-up time. This makes it unsuitable for use in cars. Phosphoric-acid systems are likely to fall out of favor because they are less efficient than other fuel cells and require a toxic gas as their catalyst.
Direct Methanol Fuel Cell (DMFC)
Methanol fuel cells are comparable to a PEMFC in regards to operating temperature but are not as efficient. Also, the DMFC requires a relatively large amount of platinum to act as a catalyst, which makes these fuel cells expensive.
Reversible Fuel Cells
A reversible fuel cell combines two methods of energy production, uniting one of the previous fuel cell types with a solar or a wind generator. Like all other fuel, it produces energy and water vapor, but the water is then stored for later. During times of high wind or solar activity, the other side of the system produces power.
Some of that power can then be run back through the fuel cell to convert the stored water back to oxygen and hydrogen fuel through electrolysis. This system boasts very high efficiency, but is also massively complex and expensive to produce.