What is LP Gas?
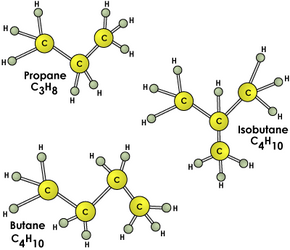
There are two LP gases that can be stored in liquid form with only moderate pressurization -- propane and butane. Isobutane, which has the same simple chemical formula as butane but has a different chemical structure, is also used. Usually, butane and isobutane are mixed with propane in various proportions, depending on the intended use of the fuel.
Propane is particularly useful as a portable fuel because its boiling point is
Advertisement
-44 F (-42 C). That means that even at very low temperatures, it will vaporize as soon as it is released from its pressurized container. This results in a clean-burning fuel that doesn't require a lot of equipment to vaporize it and mix it with air. A simple nozzle will suffice.
Butane's boiling point is approximately 31 F (-0.6 C), which means it will not vaporize in very cold temperatures. This is why butane has more limited uses and is mixed with propane instead of being used by itself.
A single pound of propane can generate 21,548 BTU (British Thermal Units) of energy, while butane can produce 21,221 BTU per pound [ref]. For comparison, here is how LP Gases stack up to other fuels in terms of energy:
- Propane: 21,500 BTU per pound
- Butane: 21,200 BTU per pound
- Gasoline: 17,500 BTU per pound
- Coal: 10,000 BTU per pound
- Wood: 7,000 BTU per pound
In the next section, we'll find out where LP gas comes from.