Directly or indirectly, nearly all life on Earth is solar-powered.
Plants convert sunlight into organic compounds that, when consumed by other life, pass on the sun's energy to the rest of the food web. As humans, we access this stored energy through digestion and by burning raw or processed plants. Petroleum is just long-dead organic matter transformed by geological forces, and first-generation biofuels are ginned up from corn, sugar cane and vegetable oil [source: The New York Times].
Advertisement
Unfortunately, petroleum is as packed with environmental and security problems as it is energy, and first-generation biofuels -- which are refined by burning other fuels -- fall well short of carbon neutrality. Worse, as global food crops literally lose ground to biofuel production, mounting scarcity drives up food prices, hunger and political instability [source: The New York Times].
But what if there were a way to have our rice and burn it, too? What if we could derive energy from crops without killing them, or generate power using plants and land not needed for food, all through the power of microbes? That's the idea behind plant-microbial fuel cells (PMFCs).
When it comes to making life work, plants might get all the good press, but it's the much-maligned microbe that holds the food chain together. Specifically, cyanobacteria help form its base; gut microbes help us digest food from it; and soil bacteria turn the resulting waste into nutrients plants can use.
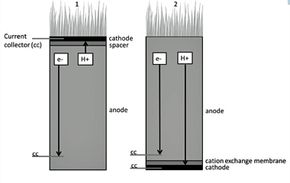
For decades, researchers have dug around for possible ways to draw power from this microbial metabolism. By the 1970s, their efforts began bearing fruit in the form of microbial fuel cells (MFCs) -- devices that generate electricity directly from a chemical reaction catalyzed by microbes [source: Rabaey and Verstraete]. MFCs offer renewable, low-power options for monitoring pollutants, cleaning and desalinating water, and powering remote sensors and instruments.
There's a catch, of course: MFCs only function as long as they have something to nosh on -- typically, organic material in the wastewater [sources: Deng, Chen and Zhao; ONR]. Researchers realized they could deliver that waste -- an unending, solar-powered buffet of it -- directly to soil microbes from plants themselves, and the seed of an idea was planted.
By 2008, researchers were publishing papers announcing the first of these plant-powered MFCs, and the potential grew increasingly clear [sources: Deng, Chen and Zhao; De Schamphelaire et al.; Strik et al.]. Using this scalable technology, villages and farms in developing countries could become self-sufficient, while industrialized nations could reduce their greenhouse footprints by drawing power from wetlands, greenhouses or biorefineries [sources: Doty; PlantPower].
PMFCs, in short, are a newer, greener spin on "power plants" -- maybe.
Advertisement